"Click"-Chemistries: CuAAC and INAIC Reactions
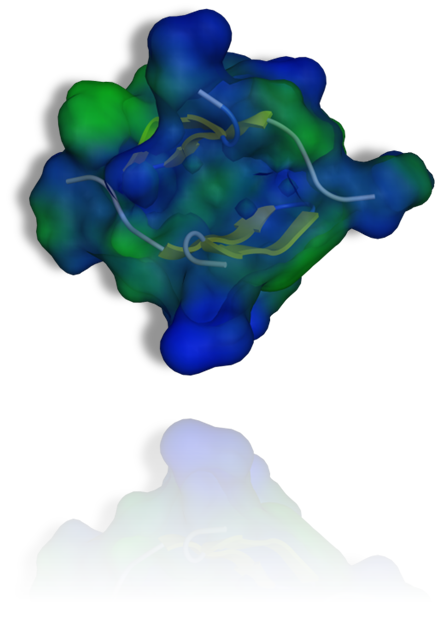
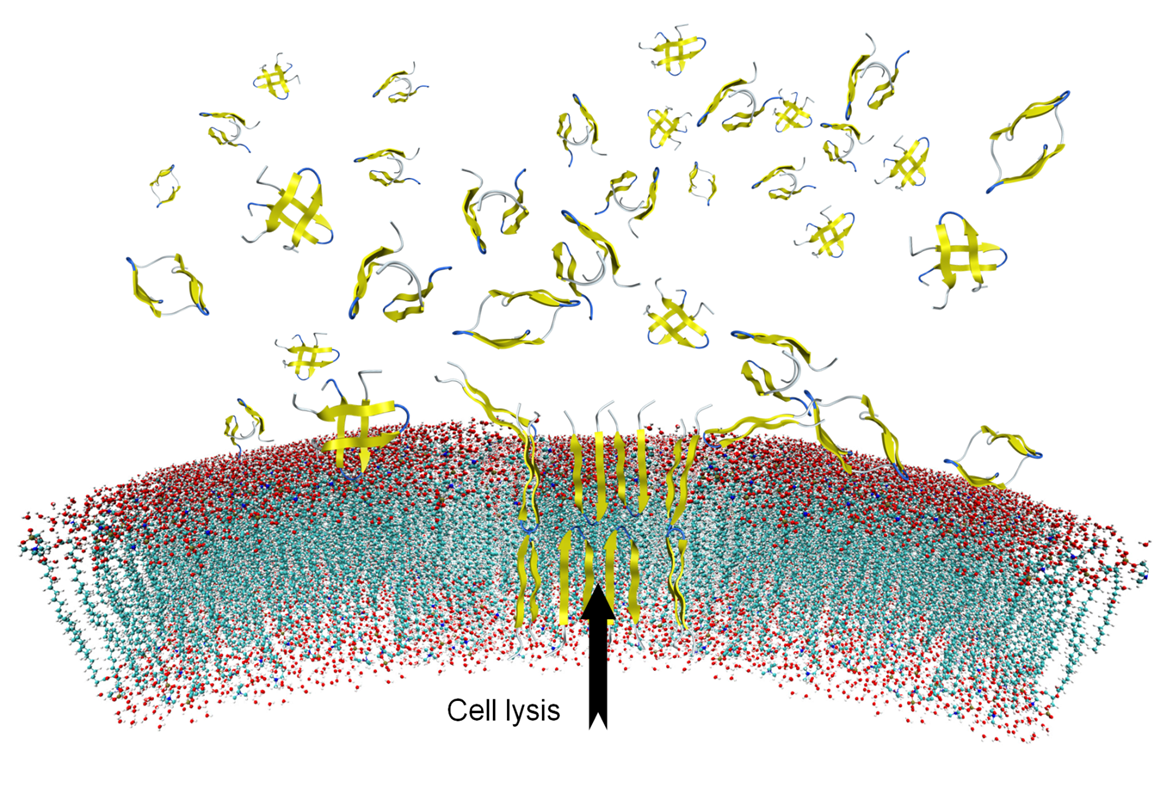
The CuAAC-click reaction, which we was introduced in 2001, has become the Royalty amongst click reactions. CECB uses CuAAC for click assembly of molecular LEGO using functional alkyne and azide modified peptides and proteins. For example the triazole analogue of the tachyplesin 1 was prepared in this manner and was shown to be a selective functional dimer (see figure) lysing only bacterial membranes.
CECB is working on development of a range of intramolecular multicomponent click reactions. These are the so called intramolecular N-acyl iminium cascade (INAIC)-reactions, which proceed quantitatively in synthesis of combinatorial libraries and convert easily assembled peptides into chiral small-molecule scaffolds.
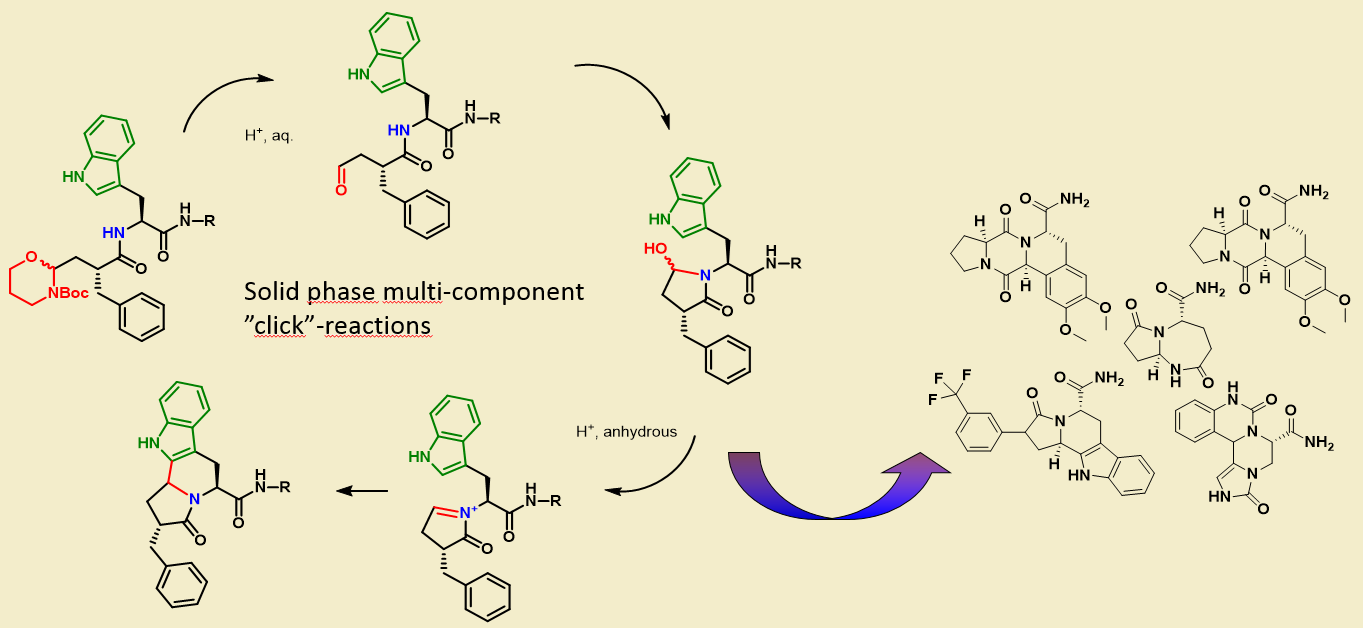
The products have great potential as ligands for receptors and can tap into the great diversity of amino acid derivatives currently available. The INIAC reaction is initiated by intramolecular nucleophilic attack of an amide bond on a proximate aldehyde. The formed hydroxylactam looses water and forms highly reactive N-acyl iminium ions. These collapse in yet another intramolecular reaction with side-chain C-, N-, S- or O-nucleophiles.
