Protein Function and "Click" Chemistry
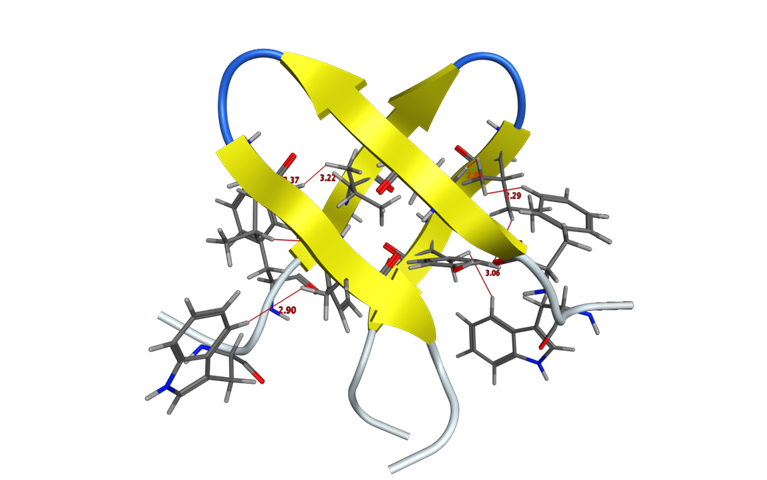
At CECB we are interested in the subtleties of molecular recognition in the transmission of biological function. An example of this is our work with Tachyplesin I analogs, which were irreversibly locked by click chemistry replacement of disulfide bonds. This allowed us to measure the formation of dimers by 1H-NMR and suggested a mechanism for antibacterial selectivity.
Dimerization and oligomerization of G-protein coupled receptors are involved in regulation of the signal transduction. In order to be able to study receptor signaling we are attempting to express proteins and protein fragments with genetic code reprograming to introduce alkynes and azides genetically and site-specifically in the protein. The aim is to be able to click-activate protein function and to establish protein-protein dimerization irreversibly. Internalization of receptors and dimerization dependent signaling is investigated
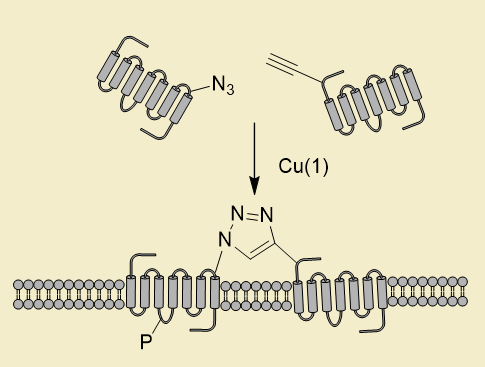
The interface in receptor dimerization allows the azide and alkyne to be incorporated at proximate residues which are in direct contact during dimerization.
