Energy Conversion Interfaces – Scott Group
A revolution is taking place in the way chemicals and energy flow through society.
Since the industrial revolution, fossil fuels have served as a source of energy, releasing CO2, and as the starting materials for most of our chemicals. The green transition taking place now will turn this on its head: renewable electricity from wind and solar will serve as the energy source needed to build all the chemicals we need from the sustainable starting materials of air, water, and CO2. Electrocatalysis, the interdisciplinary field describing how electrical energy can drive chemical reactions at the interface between a solid electrode and a fluid reaction medium, is the science at the core of this revolution. This includes the reactions that generate hydrogen (H2) and oxygen (O2) from water in an electrolyzer as well as emerging technologies that, for example, directly convert CO2 to high-value chemical feedstocks like ethylene, a gas which is a precursor to many types of plastic.
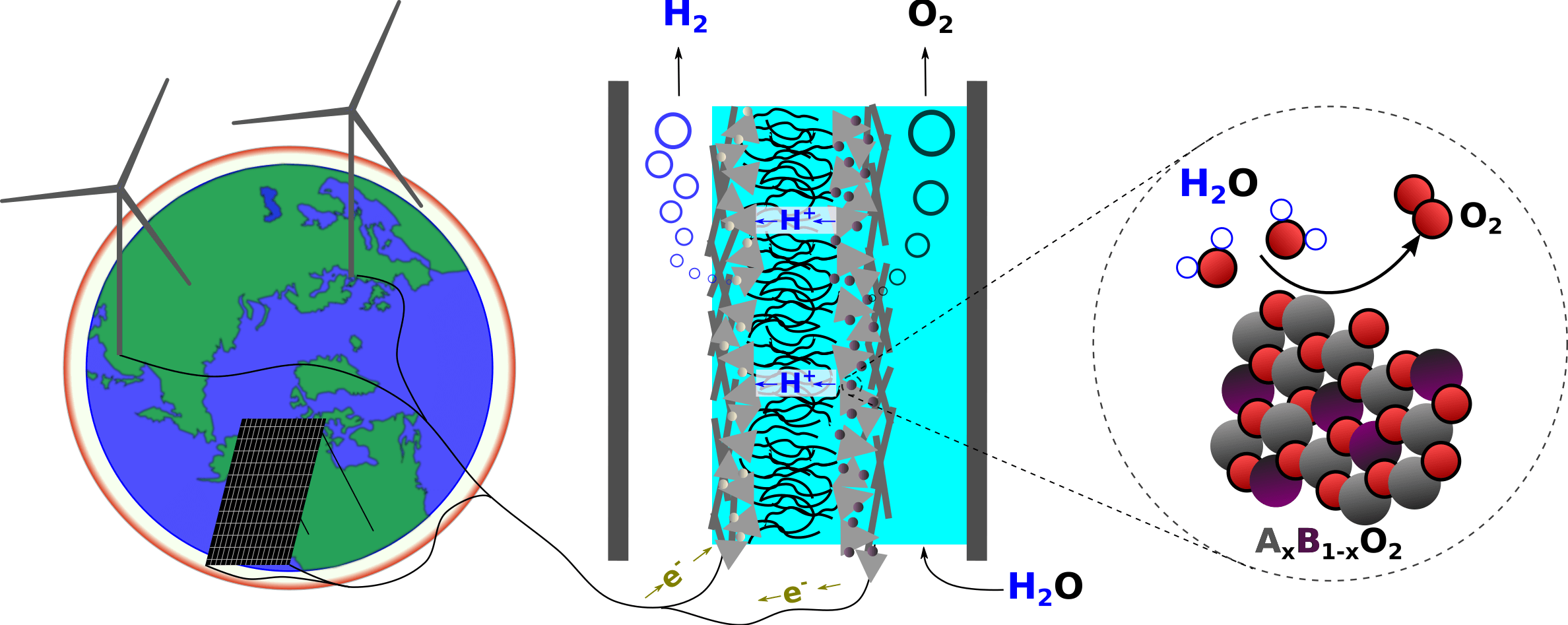
In the Scott group, we are fascinated in general by the processes that convert one type of energy to another – and specifically the electron transfer step at the core of any electrochemical reaction. We strive for fundamental understanding of the mechanisms of these reactions and the interfaces at which they occur. We will, for example, detect products real time by mass spectrometry and track the interface in-situ by UV-vis spectroscopy. We integrate data science and machine learning into our approach, and develop open source software packages for the benefit of the research community.
We work closely with the Rossmeisl, Jensen, and Pittkowski groups in the Center for High-Entropy Alloy Catalysts (CHEAC) to discover improved electrode materials and thus accelerate the green transition.
The group is led by Soren B. Scott, tenure-track assistant professor at the Department of Chemistry, University of Copenhagen.
Finding a scaleable acid-stable water oxidation catalyst
Hydrogen is certain to play a role on a terawatt scale (720 GW by 2030 and 3.7 TW by 2050 according to the IEA), and the industrial bottleneck for green hydrogen is the ability to install energy-efficient water electrolyzers quickly and at scale. In the best available technology for producing green hydrogen from intermittent renewable electricity, polymer electrolyte membrane (PEM) electrolyzers, most of the energy lost today is due to water oxidation on the anode by the oxygen evolution reaction (OER), 2 H2O O2 + 4 (H+ + e-), and this reaction at present requires the extremely rare material iridium oxide (IrO2) as electrocatalyst.
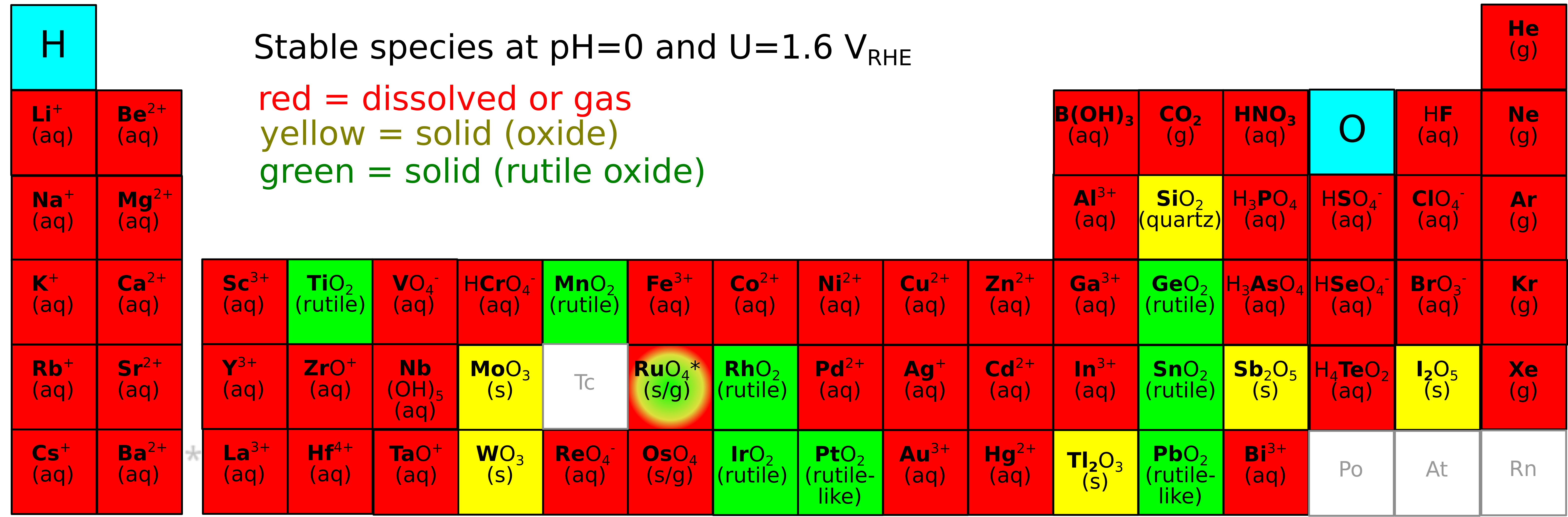 Replacing iridium is challenging due to the instability of most elements in the acidic and oxidative conditions at the PEM anode. Of the stable and abundant elements, none are good catalysts in their pure form. However, these elements form a multi-dimensional composition space of high-entropy oxides (mixes of four or more metals plus oxygen) which is likely to contain active, stable, and scalable PEM electrolyzer catalysts. Our group will search this composition space using electrodeposition synthesis, EC-MS testing, in-situ spectroscopy, and machine learning algorithms, with an emphasis on determining how the electrocatalytic mechanism varies with composition.
Replacing iridium is challenging due to the instability of most elements in the acidic and oxidative conditions at the PEM anode. Of the stable and abundant elements, none are good catalysts in their pure form. However, these elements form a multi-dimensional composition space of high-entropy oxides (mixes of four or more metals plus oxygen) which is likely to contain active, stable, and scalable PEM electrolyzer catalysts. Our group will search this composition space using electrodeposition synthesis, EC-MS testing, in-situ spectroscopy, and machine learning algorithms, with an emphasis on determining how the electrocatalytic mechanism varies with composition.
Novel in-situ methods
Discovery and optimization of the best electrocatalysts for water oxidation, or any other reaction, is facilitated by knowing how the reaction proceeds at an atomic level. Likewise, improving the stability of electrocatalysts is facilitated by knowing how degradation proceeds at an atomic level. Detecting the reaction products real-time with high sensitivity is one way to do this. With electrochemistry – mass spectrometry (EC-MS) we accurately measure the rate of any gas-producing electrochemical reaction including water splitting to O2 and H2 and carbon dioxide reduction to C2H4, for example. We can also probe electrocatalyst surface reactivity for other reactions including biomass valorization with stripping experiments, which release sub-monolayer amounts of CO2. While quantification of the reaction products can be used to infer insight in the electrocatalytic intermediates, actually observing and them as a function of potential will bring more insight to the mechanism. For this, we will build and use in-situ UV-Vis (spectroelectrochemistry, SEC) to observe and quantify electrocatalytic intermediates.
Open-source python package
The data generated from the in-situ methods described above is often complex, coming from different instruments. Often, the datasets need to be lined up in time before they can be analyzed together, which can be tedious. Furthermore, the presentation of experimental data from these techniques is not standardized, and the data is rarely shared in an easily usable way as necessary for open science. We have developed the in-situ experimental data tool (ixdat), an open-source python package, to help counter these problems. We plan to expand this as a platform for experimental data for in-situ techniques in general, and as a database system to connect experimental and computational data on electrocatalysis in a shared easy-to-use system.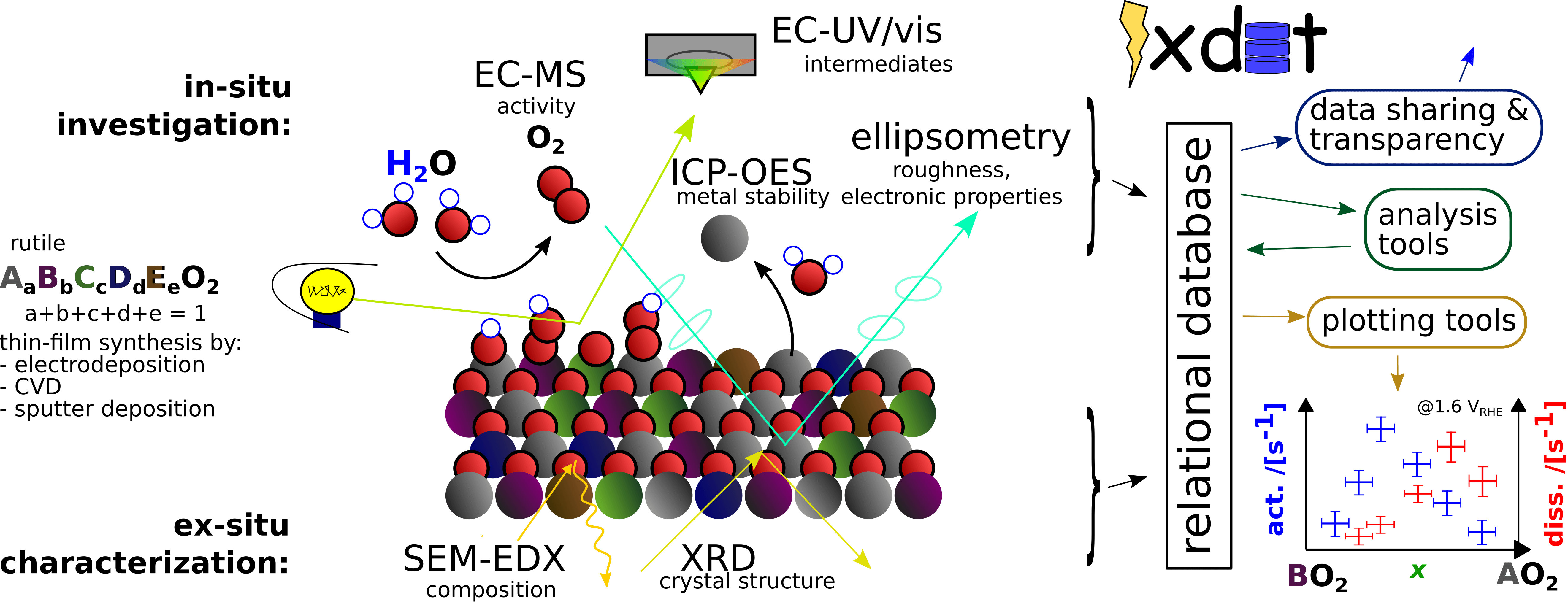
- Soren B. Scott1, Reshma R. Rao1, Choongman Moon, Jakob E. Sørensen, Jakob Kibsgaard, Yang Shao-Horn and Ib Chorkendorff. The low overpotential regime of acidic water oxidation part I: The importance of O2 detection. Energy and Environmental Science, 15, 1977-1987, 2022
https://doi.org/10.1039/d1EE03914h - Soren B. Scott, Jakob E. Sørensen, Reshma R. Rao, Choongman Moon, Jakob Kibsgaard, Yang Shao-Horn and Ib Chorkendorff. The low overpotential regime of acidic water oxidation part II: Trends in metal and oxygen stability numbers. Energy and Environmental Science, 15, 1988-2001, 2022
https://doi.org/10.1039/D1EE03915F - Stephanie A. Nitopi1, Erlend Bertheussen1, Soren B. Scott, Xinyan Liu, Albert K. Engstfeld, Sebastian Horch, Brian Seger, Ifan Stephens, Karen Chan, Christopher Hahn, Jens K. Nørskov, Thomas Jaramillo, and Ib Chorkendorff. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chemical Reviews. 119, 7610-7672, 2019
https://doi.org/10.1021/acs.chemrev.8b00705 - James Murawski, Soren B. Scott, Reshma Rao, Chris Zalitis, James Stevens, Gareth Hinds, Ifan E.L. Stephens. Benchmarking stability of IrOx in acidic media under O2 evolution conditions: a review. Johnson Matthey Technology Review, 2023, Fast-track article
https://doi.org/10.1595/205651323X16848455435118 - Zamaan Mukadam, Sihang Liu, Angus Pedersen, Jesús Barrio, Sarah Fearn, Maria-Magdalena Titirici, Soren B. Scott*, Ifan E. L. Stephens*, Karen Chan, Stefano Mezzavilla*. Furfural Electrovalorisation to Jet Fuels Using Single-atom Molecular Catalysts. Energy Environmental Science, 16, 2934-2944 2023
https://doi.org/10.1039/D3EE00551H - Luca Silvioli, Anna Winiwarter, Soren B. Scott, Ivano E. Castelli, Poul G. Moses, Ib Chorkendorff, Brian Seger, and Jan Rossmeisl. Rational Catalyst Design for Higher Propene Partial Electrooxidation Activity by Alloying Pd with Au. Journal of Physical Chemistry C, 126, 34, 14487–14499, 2022
https://doi.org/10.1021/acs.jpcc.1c10095 - Soren B. Scott, Jakob Kibsgaard, Peter C.K. Vesborg, and Ib Chorkendorff. Tracking oxygen atoms in electrochemical CO oxidation – Part II: Lattice oxygen reactivity in oxides of Pt and Ir. Electrochimica Acta, 374, 137844, 2021
https://doi.org/10.1016/j.electacta.2021.137844 - Soren B. Scott, Jakob Kibsgaard, Peter C.K. Vesborg, and Ib Chorkendorff. Tracking oxygen atoms in electrochemical CO oxidation – Part I: Oxygen exchange via CO2 hydration. Electrochimica Acta, 374, 137842, 2021
https://doi.org/10.1016/j.electacta.2021.137842 - Soren B. Scott, Thomas V. Hogg, Alan T. Landers, Thomas Maagaard, Erlend Bertheussen, John C. Lin, Ryan C. Davis, Jefferey W. Beeman, Drew Higgins, Walter S. Drisdell, Apurva Mehta, Brian J. Seger, Thomas F. Jaramillo, and Ib Chorkendorff. Absence of Oxidized Phases in Cu under CO Reduction Conditions. ACS Energy Lett. 4, 803 - 804, 2019
https://doi.org/10.1021/acsenergylett.9b00172 - Daniel B. Trimarco1, Soren B. Scott1, Anil H. Thilsted, Jesper Y. Pan, Thomas Pedersen, Ole Hansen, Ib Chorkendorff, and Peter C.K. Vesborg. Enabling real-time detection of electrochemical desorption phenomena with sub-monolayer sensitivity. Electrochimica Acta, 268, 520-530, 2018
https://doi.org/10.1016/j.electacta.2018.02.060
